Article
Why eClinical architectures have reached their limits, and a new category is emerging
Clinical research has changed. But the existing eClinical environment has not developed at the same pace as clinical research.
Today's research operates under very different conditions than even a decade ago. Studies are more complex, protocols evolve during execution, the patient journey involves more touchpoints, and data is generated continuously across sites, systems, and participants.
Regulatory expectations increasingly focus on traceability and proactive oversight rather than retrospective control.
Yet the technology landscape supporting clinical research has largely remained built on older assumptions. Many organizations still rely on fragmented system architectures with limited data reuse and delayed visibility. This growing mismatch has created structural friction that can no longer be solved through incremental optimization.
The current system landscape of point solutions and eClinical environments
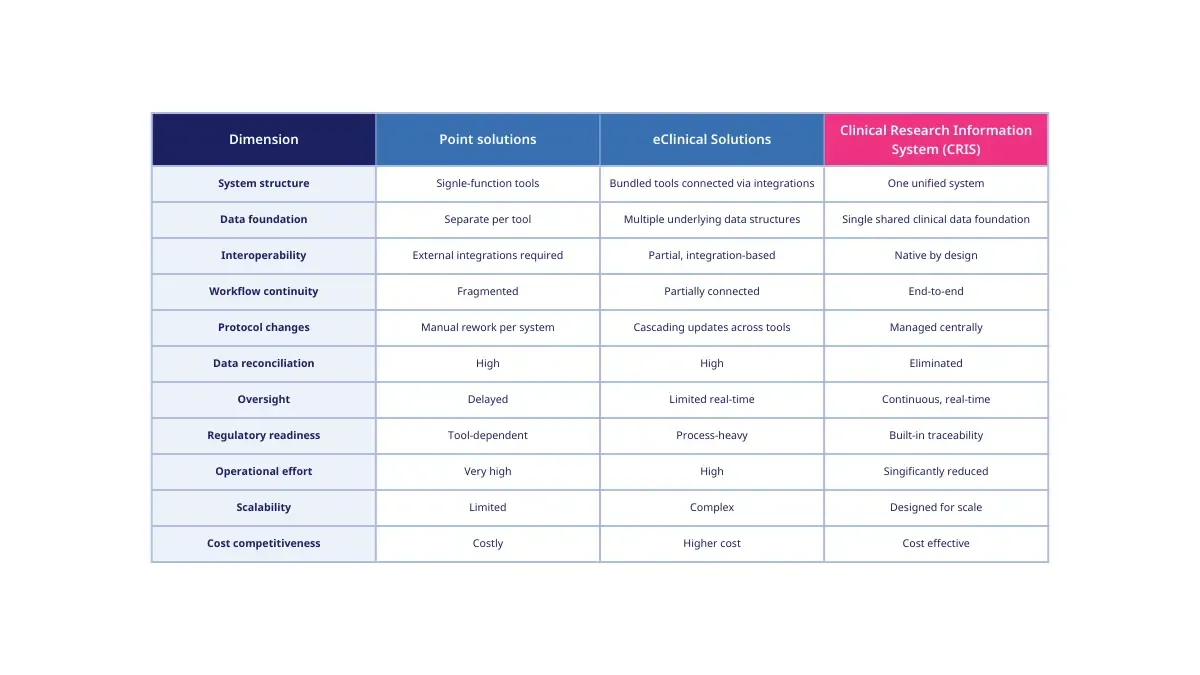
Clinical research technology operates under two dominant models.
Point solutions address individual functions such as Electronic Data Capture (EDC), Electronic Trial Master File (eTMF,) patient-reported outcomes, or trial supply management. Each tool is optimized for a specific task but largely operates independently.
eClinical solutions bundle several of these point solutions on a platform offered by a single vendor. While often presented as integrated suites, they typically consist of mainly independently developed systems connected through interfaces and integrations.
This model became the industry standard through gradual expansion rather than architectural design. As new requirements emerged, new tools were added, to the existing platforms, requiring additional integrations and manual processes.
The structural limits of eClinical solutions
The core challenge of eClinical environments is not functionality. It is architecture.
Even when integrations exist, eClinical solutions usually rely on separate data structures per module, system-specific configuration and logic, fragmented workflows and permission models, and periodic synchronization rather than continuous data consistency.
As a result, operational effort shifts from study execution toward system coordination. Protocol changes trigger depended updates across tools. Data reconciliation becomes a permanent activity. Oversight depends on delayed consolidation rather than real-time visibility.
Why eClinical solutions cannot become Clinical research Information System (CRIS)
eClinical solutions cannot evolve into Clinical Research Information Systems because they were not designed around a unified data foundation. They are built by connecting independently developed tools, each with their own internal logic and data structures.
Adding integrations, harmonization layers, or shared interfaces does not change this underlying fragmentation. eClinical solutions connect tools; CRIS redesigns the foundation.
Introducing a new category: Clinical Research Information System (CRIS)
The structural limits of point solutions and eClinical environments have led to the emergence of a new system category: Clinical Research Information Systems.
CRIS is not a rebranding of existing tools and not an extension of the eClinical suite model. It represents a fundamentally different approach to how clinical research systems are designed.
A Clinical Research Information System is a unified clinical research environment built on a shared data foundation achieving all assets of clinical data interoperability. The outcome is that all study functions operate coherently by design, allowing the management off all relevant clinical research processes within one single system.
What defines a Clinical Research Information System
A CRIS is characterized by a single shared clinical data foundation across the study lifecycle, consistent data structures and semantics across all functions, native workflow, continuous data availability and analysis readiness.
This allows real-time management, collaboration, and knowledge generation across the processes and teams. The built-in traceability and audit readiness ensure full compliance at any time during the study lifecycle.
Why the distinction matters in practice
With oomnia, Wemedoo has introduced a concrete example of how a Clinical Research Information System can work in practice.
oomnia was designed around CRIS principles, with all system functions operating on a shared, structured clinical data foundation. This makes clinical data interoperability native by design, with complexity addressed at the core of the system.
Explore how oomnia brings CRIS architecture into real-world use.

January 6, 2026
Clinical trial trends in 2025
Blogs

September 18, 2025
The hidden cost of disconnected systems in biotech and the path to streamlined trials
Blogs

August 6, 2025
Accessing better technology for clinical trials
Blogs